Abstract
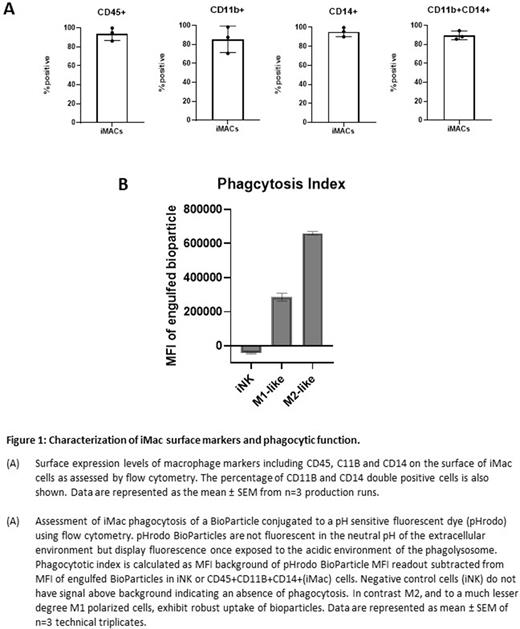
Human induced pluripotent stem cells (hiPSCs) exhibit unlimited self-renewal potential and have the capacity to differentiate into somatic cell derivatives of all three germ layers, making them an ideal source for generation of cellular therapies. hiPSCs also address the inherent variability that arises from the use of patient- or donor-sourced cells which can lead to drug product inconsistencies that impact safety, efficacy, and therapeutic utility. To leverage these attributes, we have established a platform that enables multiplexed engineering of hiPSCs at the single-cell level and have developed a proprietary stage-specific differentiation protocol to support definitive hematopoiesis for the derivation of CD34+ hematopoietic progenitor (iCD34) cells. We have previously shown that these iCD34 cells exhibit efficient differentiation and expansion to lymphoid lineages, including Natural Killer (NK) and αβ T-cells, and the potential to develop B-cell and regulatory T-cells (Tregs) based cellular therapies. Additionally, iCD34 cells can efficiently give rise to myeloid populations including myeloid progenitor cells and macrophages, the latter being further modified with a CAR motif for various therapeutic opportunities. To this end, we have established a process for differentiating our engineered iCD34 cells into myeloid populations. Leveraging key aspects of definitive hematopoiesis, the iCD34 cells expand and differentiate into myeloid progenitor cells and into functional macrophages (iMacs). iMacs have a typical macrophage morphology with elongated amoeboid or rounded cell shape, irregular cell borders, oval- or kidney-shaped nucleus, cytoplasmic vesicles and numerous delicate cytoplasmic processes and uniformly express known macrophage markers including CD14 (95%), CD11b (86%) and CD45 (93%) (Figure 1A). They are also capable of executing on key macrophage functions such as phagocytosis (Figure 1B) and the secretion of specific cytokines. Macrophages acquire further specification based on environmental stimuli and are broadly classified into M1-like or pro-inflammatory and M2-like or anti-inflammatory populations. Similarly, iMacs can express markers of M1 or M2 polarized macrophages and demonstrate phenotype specific phagocytic behavior with M2 polarized cells exhibiting substantially more phagocytosis compared to M1 or negative control cells (Figure 1B). In preliminary in vivo studies, iMacs show trafficking to multiple compartments and can infiltrate and establish residence in multiple tissues. Furthermore, preliminary phenotyping data shows iMacs engineered with (chimeric antigen receptor) CAR or Fc receptor such as high-affinity noncleavable (hnCD16) or CD64 can uniformly express the engineered transgene (>90%).
Collectively, the data further confirm that the iCD34 cells generated from our hiPSC platform, are multipotent hematopoietic progenitor cells and can efficiently expand and differentiate into lymphoid and myeloid lineages with functional immune cell capacity. In addition to the generation of multiplexed engineered iT and iNK cells, the ability to differentiate multipotent iCD34 cells to the myeloid lineage enables the use of this versatile product platform to develop highly engineered off-the-shelf macrophage-based cellular therapies with the potential for the treatment of many pathological conditions, including antigen-specific targeting through CAR or Fc receptor to induce antibody-dependent cellular phagocytosis.
Disclosures
Labarge:Fate Therapeutics Inc.: Current Employment. Sims:Fate Therapeutics Inc.: Current Employment. Sandoval:Fate Therapeutics Inc.: Current Employment. Bagri:Fate Therapeutics Inc.: Current Employment. Witty:Fate Therapeutics, Inc.: Current Employment. Valamehr:Fate Therapeutics: Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal